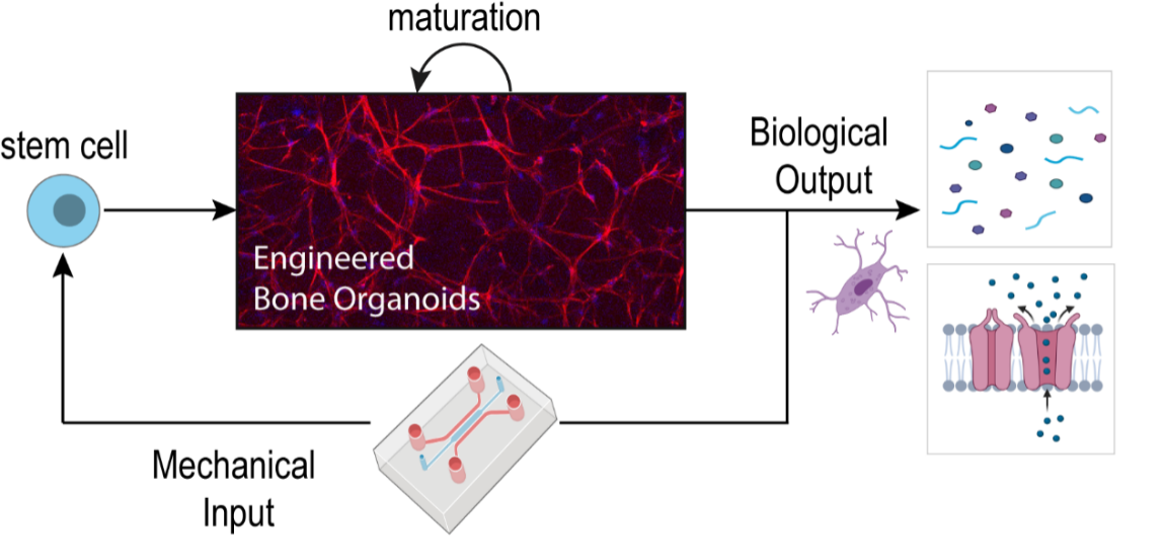
Human Bone-Organoid-on-Chip
Modelling human biology in microphysiological in vitro systems will enrich our mechanistic understanding of human diseases and may eventually enable researchers to predict how individual patients respond to drug candidates. Despite decades of research, our knowledge about bone pathology is mostly gained from expensive animal experiments, which are often too complex to dissect the contribution of molecular and cellular factors.
In this project, we aim to develop human bone-organoid-on-chip models through microfluidic perfusion culture using bone cells embedded in a hydrogel. Microfluidic technology enables cell cultivation at microscales with exquisite control over microenvironmental cues, such as cell-cell and cell-matrix interactions. It is ideally suited for controlled delivery of physiological fluid shear stress into 3D cell cultures. With this toolbox, we seek to investigate how mechanical stimulation influences cellular behaviors and bone formation within a dynamic 3D microenvironment. Considering the 3R principles, our human bone-organoid-on-chip platform has great potential to be applied in fundamental and translational bone research with the long-term goal to replace animal experiments. One major focus of this project is to apply this in vitro tool to study the pathomechanisms of rare bone disease in collaboration with University Children’s Hospital Zurich (KSIPI) and other academic /industrial partners.
Related student project:
- Monica Müller (HS 2020, D-HEST, MSc thesis)
- Leana Bissig (FS 2021, D-HEST, MSc internship)
- Doris Zauchner (FS 2021, D-ITET, MSc thesis)
- Isabel Hui (HS 2022, D-ITET, Semester thesis)
Collaborators:
- Dr. Pei Jin Lim, Connective Tissue Unit, Division of Metabolism and Children’s Research Center, University Children’s Hospital Zurich
- Dr. Cecilia Giunta, Connective Tissue Unit, Division of Metabolism and Children’s Research Center, University Children’s Hospital Zurich
- Prof. Dr. Marianne Rohrbach, Connective Tissue Unit, Division of Metabolism and Children’s Research Center, University Children’s Hospital Zurich
- Dr. Feihu Zhao, Zinekiewicz Centre for Computational Engineering, Swansea University, UK
- Dr. Sung Sik Lee, Head of Optofluidics unit, ScopeM and Institute of Biochemistry, ETH Zurich
Acknowledgements:
We are grateful to the Swiss National Science Foundation (SNSF) National Research Programme (NRP) 79 ‘‘Advancing 3R’’ and SNSF Spark Award for the financial support.