Research
Bone is a living organ that is constantly remodeled by billions of bone cells that can sense the applied mechanical loads and coordinate bone adaptation throughout human life. Developing tools and 3D bone cell models that recapitulate human bone physiology in vitro offers the means to understand bone biology at the next level, to discover new biomarkers affecting bone remodeling and to screen potential therapeutic approaches to treat human diseases.
Our Goal
In the Biomaterials Engineering Laboratory, we aim to develop miniature in vitro models of human bone for regenerative medicine. We leverage interdisciplinary advances in developmental biology and tissue engineering to build dynamic 3D living bone cell models of in vivo-like functionality. We merge advanced biomaterials with high-resolution biofabrication and on-chip techniques to recreate the microarchitecture and function of human bone tissues. We then apply these microengineered bone organoids to study how bone cells sense and respond to mechanical signals at the molecular level.
Our Expertise
Our expertise is in the development of molecularly engineered soft biomaterials, biomimetic computer models, high-resolution biofabrication technology, tools for on-chip mechanical loading and 3D organotypic culture systems. Team members have multidisciplinary backgrounds ranging from chemistry and engineering to cell biology. The team is active in collaborating with experts with complementary expertise in bone imaging and biology.
Projects
Molecularly Engineered Biomaterials
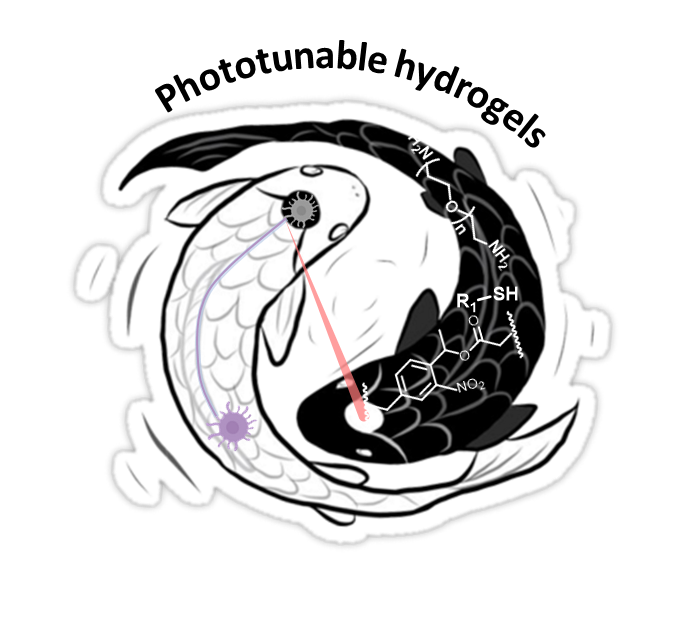
Hydrogels, water-containing crosslinked polymer networks, are widely used in tissue engineering and regenerative medicine, as their physicochemical properties resemble the native extracellular matrix (ECM) of soft tissues.
Cell-matrix Interactions
Within native tissues cells reside in the extracellular matrix (ECM). The ECM represents a complex 3D environment that stores numerous biophysical and biochemical signals.
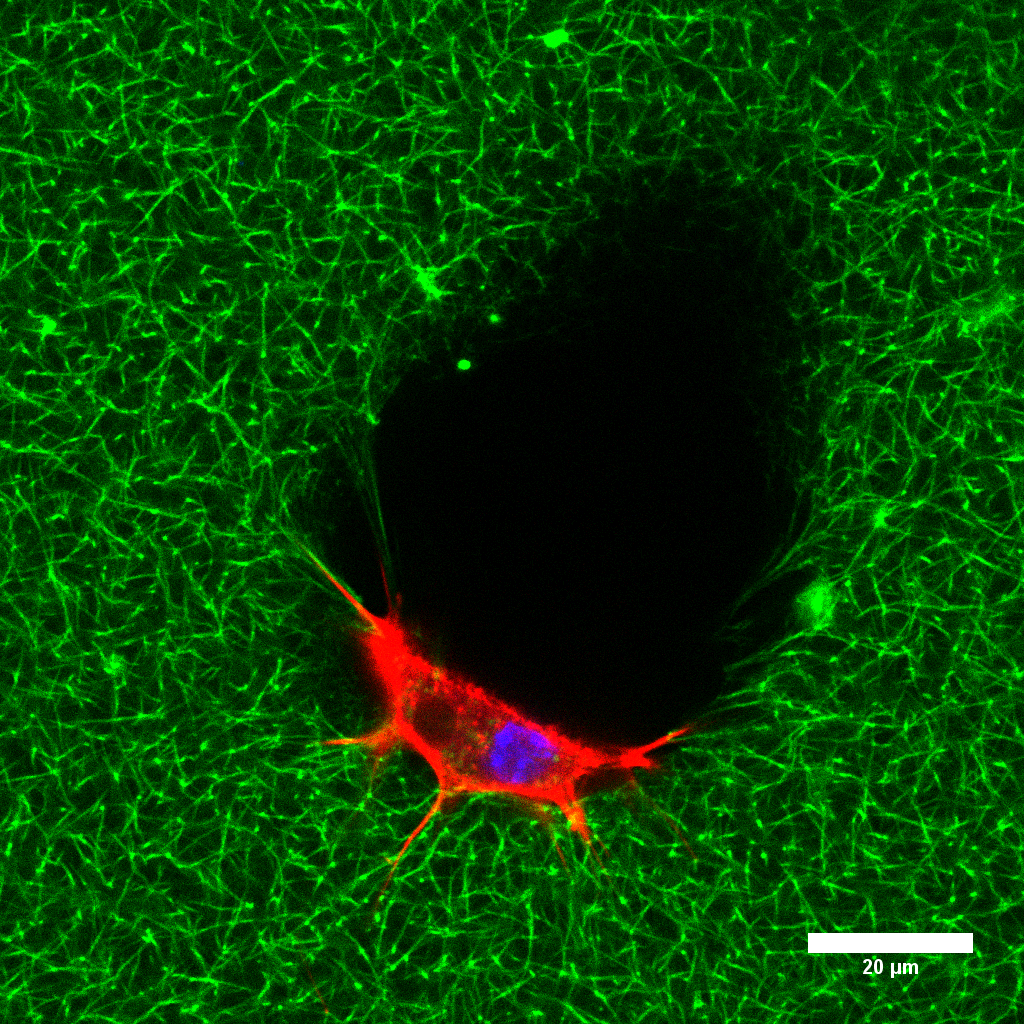
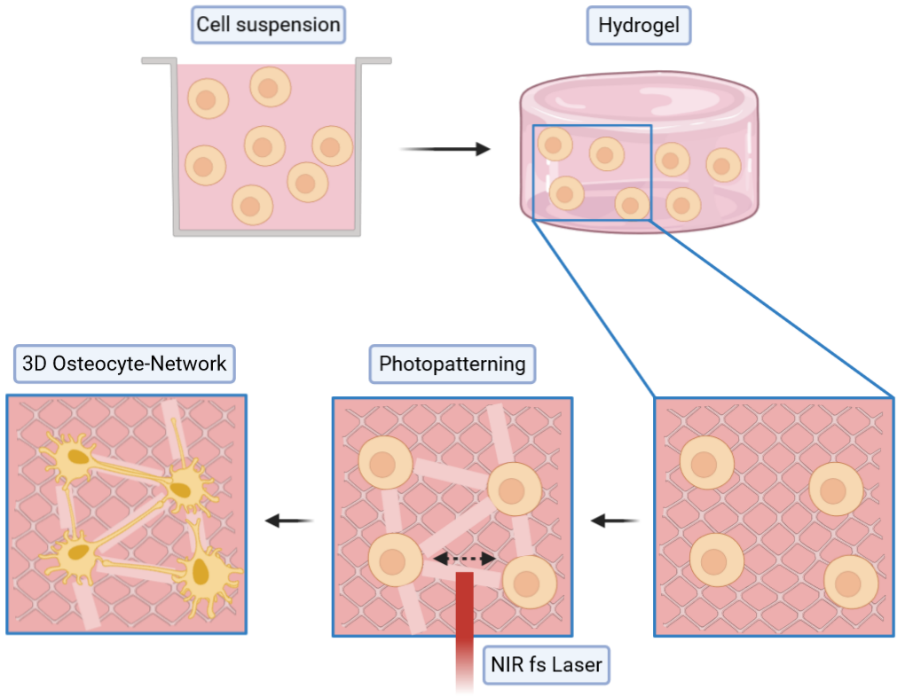
3D Microprinted Bone Cell Models
Bone itself is traversed by a complex system of cavities and channels, called the lacuna-canalicular-network (LCN). Osteocytes, mature bone cells that function as orchestrators of load-induced bone remodeling, reside within these cavities and stretch throughout the canaliculi to create a cellular network.
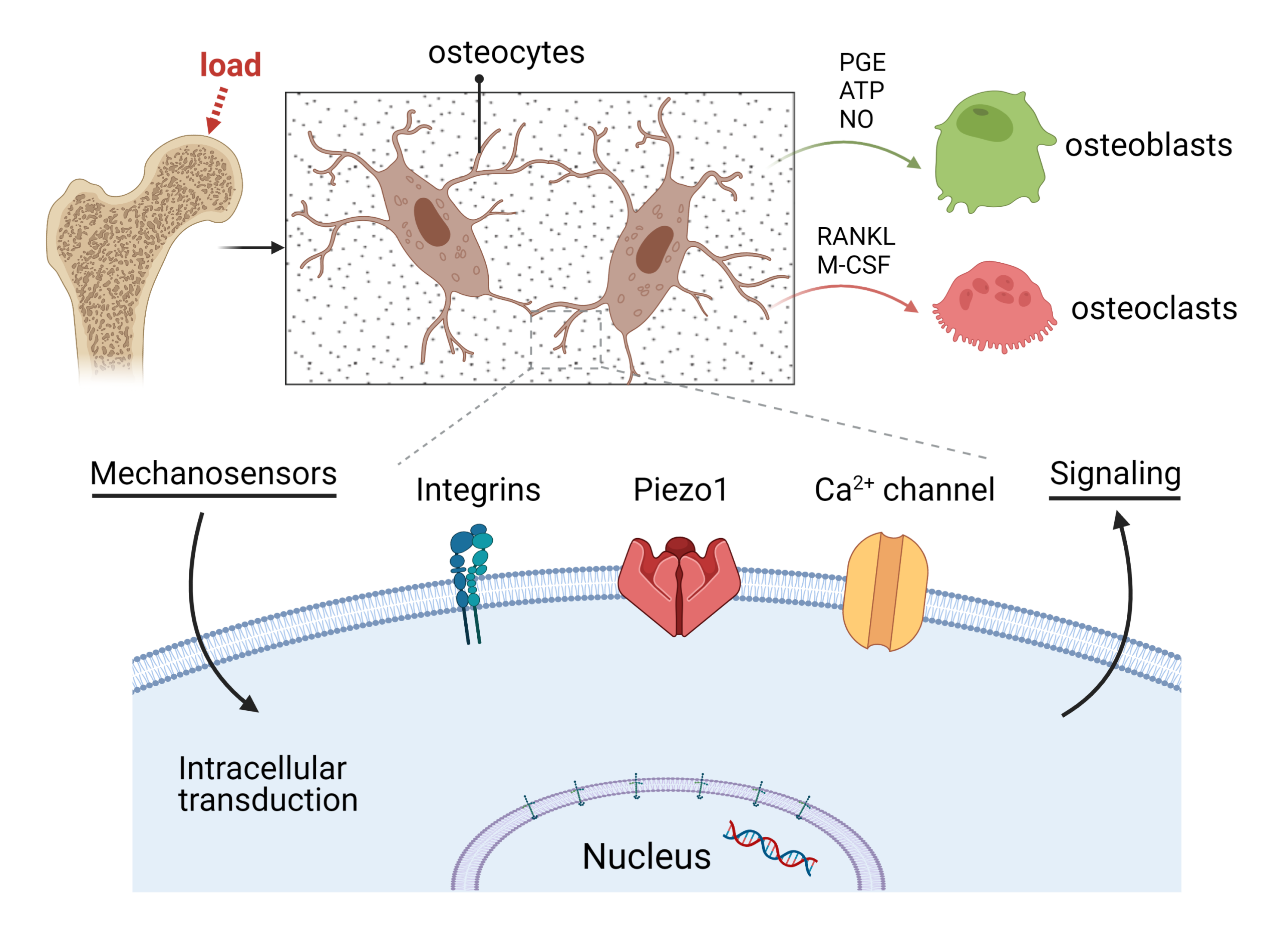
Osteocyte Mechanobiology
Osteocytes are primary cells in bone tissue. They are able to sense mechanical forces from extracellular matrix (ECM) and then transfer this biomechanical simulation into nuclear organelle and express related genes.
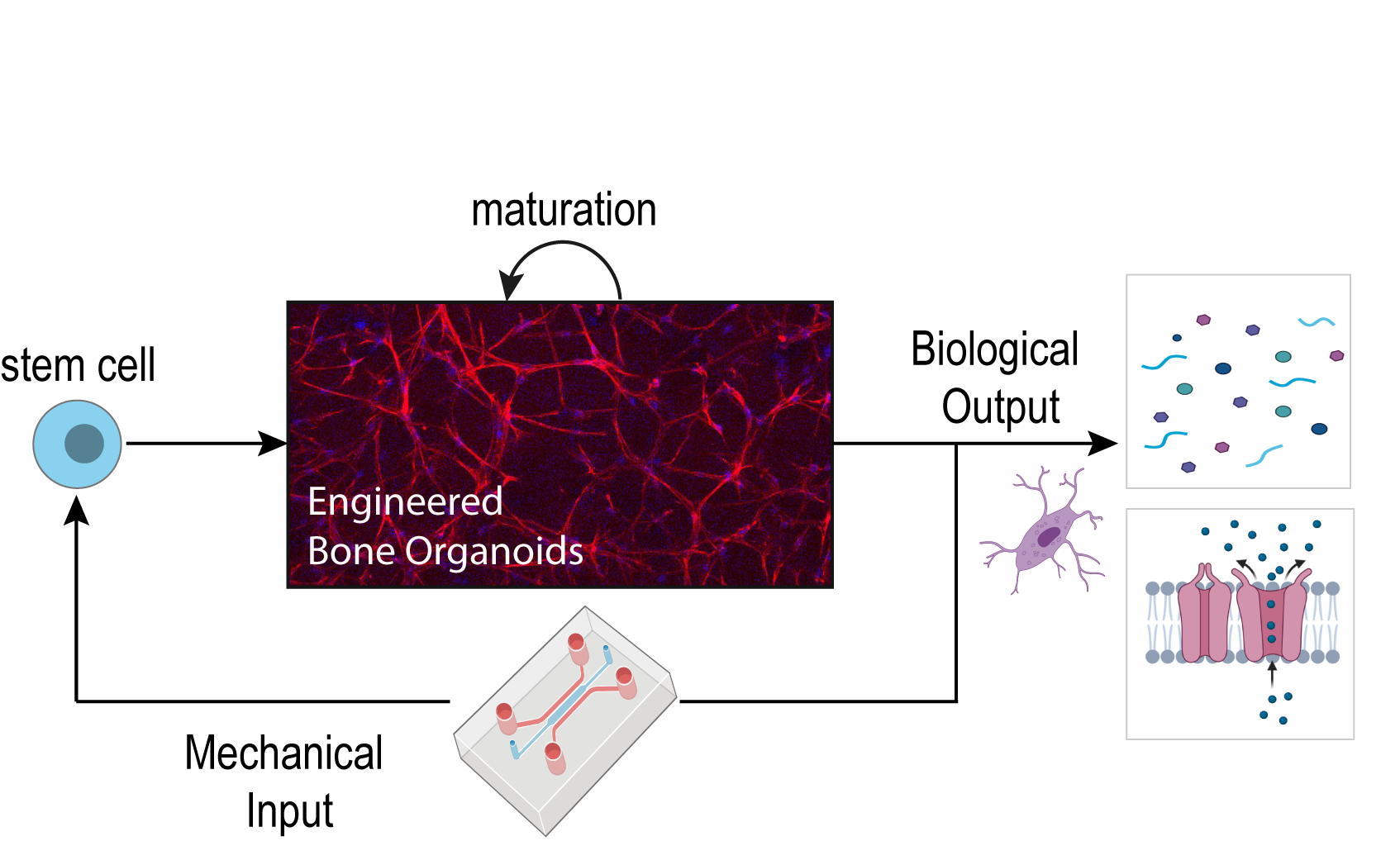
Human Bone-Organoid-on-Chip
Modelling human biology in microphysiological in vitro systems will enrich our mechanistic understanding of human diseases and may eventually enable researchers to predict how individual patients respond to drug candidates.